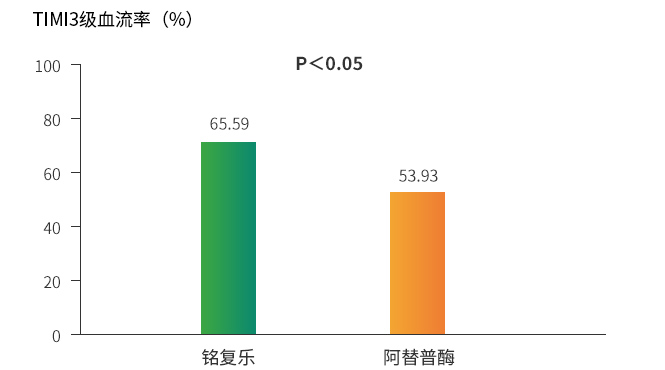
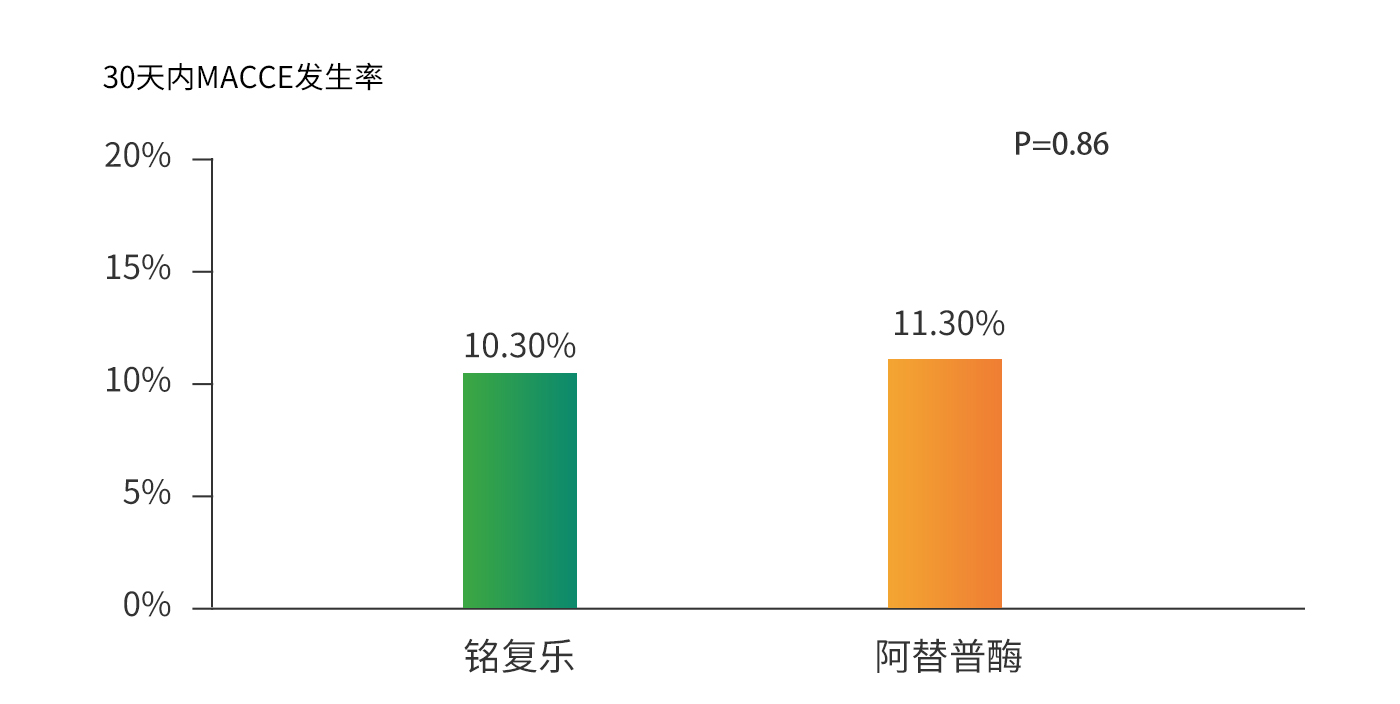
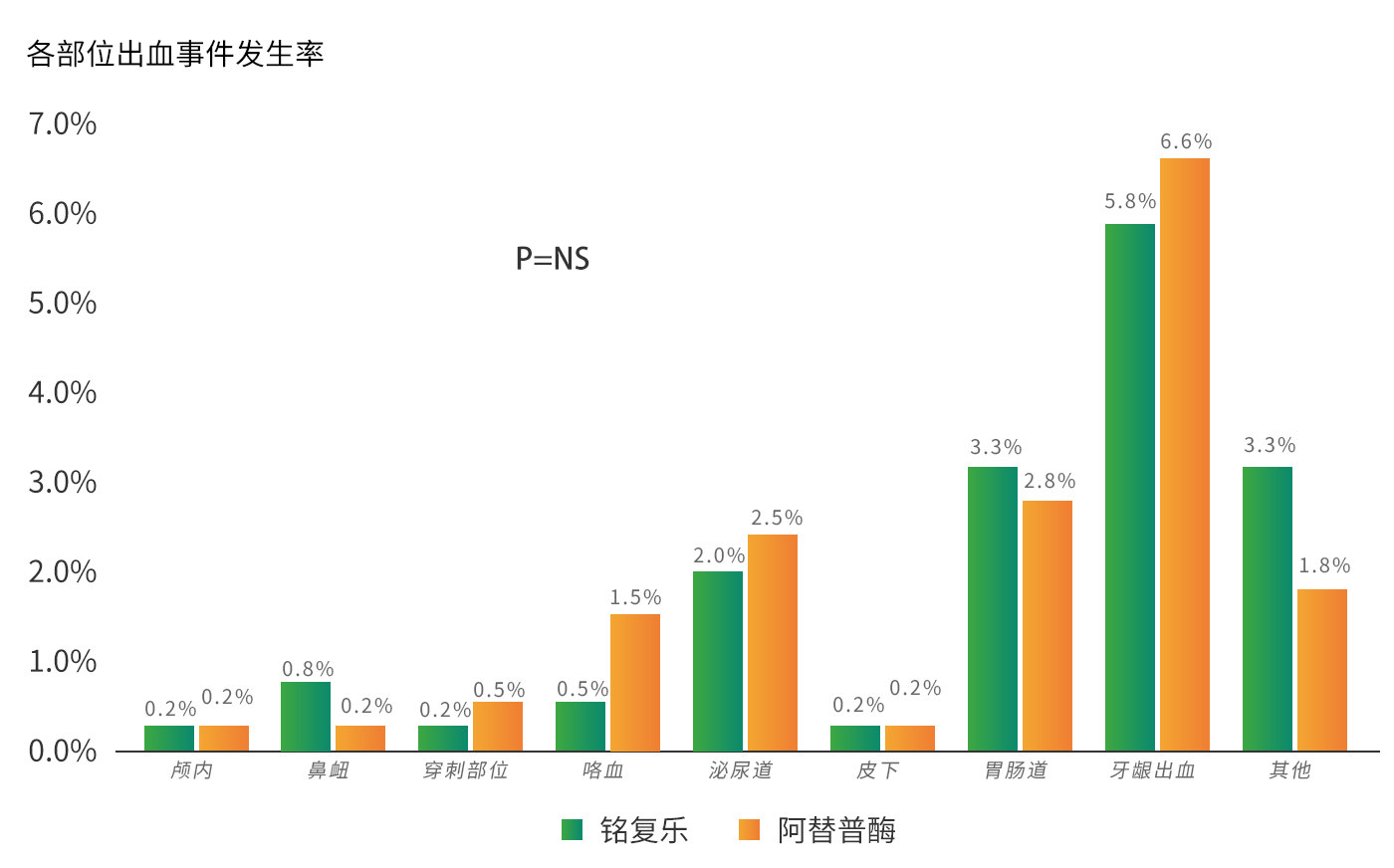
Thrombolysis patency rate of 83%
Phase II Clinical Study of Mineflex Heart Attack
Study design: a randomized, blinded, parallel, positive-controlled, multicenter clinical study enrolling 251 patients aged 18-70 years with STEMI within 12 h of onset
Primary efficacy: The patency rate of Reflow™ (rhTNK-tPA) 16 mg for 90 min infarcted blood vessels was significantly higher than that of recombinant human tissue-type plasminogen activator (rt-PA) (p<0.05)